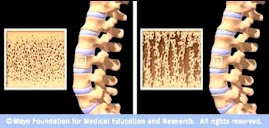
Numerous studies on sr. ranelate
found increasing BMD correlated with decreasing fracture risk (references #16
and #21-25 listed at the end of the Comb Study cited below). The French
pharmaceutical company, Servier, funded the research on sr. ranelate to market
the drug in Europe and elsewhere. Taking a drug through all the required
clinical trials takes millions of dollars and the resources of a large company
like Servier, which is present in 140 countries,
with more than 20,000 employees, including close to 3000 in Research and
Development (R&D).
Strontium
citrate is not patentable and is sold as a supplement in the U.S. and Canada. There
is no monetary incentive for a large pharmaceutical company to do research on
strontium citrate. Any research on sr. citrate is most likely to come from
universities that have obtained grants. We will continue to see small-scale
strontium citrate studies that will add to our knowledge. I do not expect to
see large-scale clinical trials involving thousands of subjects taking
strontium citrate over a period of several years. Those are the types of trials
needed to prove fracture-risk efficacy.
The
following is a review of some significant studies on strontium citrate:
In 2007, two American researchers
with SDM College of Dental Sciences in Buffalo, NY, presented
their work on osteoblasts at a dental conference. They wrote: “The data support the hypothesis that strontium citrate increases
the proliferative/alkaline phosphatase activity of human osteoblastic cells
from alveolar bone. The results validate previous research that has been done
with other forms of strontium in clinical studies and rodent calvarial cells
and indicates that strontium citrate could be a promising agent in treating
oral as well as systemic bone disorders.” The abstract of their paper is available here:
http://iadr.confex.com/iadr/2007orleans/techprogram/abstract_89231.htm