I take several supplements. Recently, I have reduced the dosage or eliminated some of them.
AOR Strontium Support II, 341 mg strontium (from citrate)/capsule
Reduced from two capsules (682 mg Sr)/day to one capsule/day for four days/week and two capsules/day for three days/week
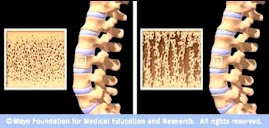
Reason: My DXA scan of August 18, 2022 showed "normal" bone density. Due to the bone strontium effect, my BMD is more likely at the osteopenia stage. Sure beats osteoporosis!
Magnesium glycinate
For about a year and a half, I took one capsule per day of Innate Vitality Magnesium Glycinate, 500 mg/capsule. The label does not give the amount of elemental magnesium, which would be 70.5 mg (14.1% of 500 mg magnesium glycinate). I think it is misleading to not provide the amount of Mg. I switched to Doctor's Best High Absorption Magnesium Glycinate Lysinate, which has 100 mg of Mg from 1000 mg magnesium lysinate glycinate chelate per tablet.
Eliminated magnesium glycinate
Reason: I was having multiple bowel movements with Doctor's Best, although not with Innate Vitality. There is Mg in my calcium supplement and my multivitamin. I also eat magnesium-rich foods. My husband is still taking Doctor's Best magnesium.
Horbaach Sublingual Methylcobalamin (Vitamin B-12), 5000 mcg/tablet
I was taking 5000 mcg/day, which is a huge amount. I knew it was a huge amount, but my husband needs that amount of B-12 to keep from getting canker sores. He got recurrent sores until he found out about B-12 for canker sores from a scientific article. More recently, I read that, in some people, B-12 can worsen acne because Propionibacterium acnes (P. acnes) need B-12. I have always had acne-prone skin.
Reduced from one tablet/day to one tablet/week. My husband still takes it daily.
Reason: Worsening acne flareups, and recommended doses are much lower than I was taking
Probiotic, Various Brands
Eliminated probiotic.
Reason: Not sure the ones I tried were helping my gas and bloating. For many years, I used Nature's Way Primadophilus Bifidus, which seemed to help my digestion. It had to be mailed overnight with a cold pack, but many vendors, such as Amazon, are not keeping it cold.
Digestive Enzymes, Various Brands
Eliminated digestive enzymes
Reason: None of the various brands made a noticeable difference for my gas and bloating. I am not lactose intolerant. If I were, I would take Lactaid, or a generic lactase supplement, as my husband does.