Strontium is safe for most people, but there are certain individuals who should not take it. These include those with any of the following disorders:
- Decreased kidney function.
- History of blood clots in the veins (venous thromboembolism, e.g., deep vein thrombosis or pulmonary embolism).
- Blood disorders that increase the risk of blood clots in the veins, e.g. antiphospholipid syndrome, factor V Leiden.
There is some evidence that strontium could slightly increase the incidence of blood clots. The tests were performed on strontium ranelate.
Strontium should also not be taken by children nor by pregnant or lactating women.
Human Skeleton

WELCOME TO STRONTIUM FOR BONES BLOG
Have you experienced negative, and even dangerous, side effects from Fosamax (alendronate), Boniva (ibandronate), Actonel (risedronate), Reclast (zoledronic acid), Prolia (denosumab), Forteo (teriparatide), Tymlos (abaloparatide), or other drugs prescribed for osteoporosis? If you have, then rest assured there is a safe, effective treatment for this condition. Strontium, primarily in the form of strontium citrate, is taken orally once a day.
Visitors to my blog can leave comments or ask questions and can remain anonymous, if they wish. Their comments are relayed to my g-mail inbox. Below each post, the number of comments for that post is cited and underlined because it is a link. By clicking on that link below any post, a window opens so that a visitor can leave a comment. Ideally, visitors leave comments on posts most relevant to their comments. All comments to my posts are moderated by me.
Browse the posts and visit the link library of references.
Visitors to my blog can leave comments or ask questions and can remain anonymous, if they wish. Their comments are relayed to my g-mail inbox. Below each post, the number of comments for that post is cited and underlined because it is a link. By clicking on that link below any post, a window opens so that a visitor can leave a comment. Ideally, visitors leave comments on posts most relevant to their comments. All comments to my posts are moderated by me.
Browse the posts and visit the link library of references.
Friday, April 17, 2009
Wednesday, April 8, 2009
A Dose-Response Study With Strontium Malonate
Osteologix, Inc. is conducting a Phase II clinical trial with strontium malonate in postmenopausal women. The study's primary objective is to compare dose-response effect of three levels of strontium malonate to placebo on bone resorption quantified by S-CTX-1 following 12 weeks of treatment. The four parallel groups in this trial will take 750 mg strontium malonate, 1000 mg strontium malonate, 2000 mg strontium malonate, 2 grams Protelos (strontium ranelate), and placebo. For more details, visit http://clinicaltrials.gov/ct2/show/NCT00409032
Tuesday, April 7, 2009
FDA Bisphosphonate Alert
Information on Bisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa)
FDA ALERT [1/7/2008] - FDA is highlighting the possibility of severe and sometimes incapacitating bone, joint, and/or muscle (musculoskeletal) pain in patients taking bisphosphonates. Although severe musculoskeletal pain is included in the prescribing information for all bisphosphonates, the association between bisphosphonates and severe musculoskeletal pain may be overlooked by healthcare professionals, delaying diagnosis, prolonging pain and/or impairment, and necessitating the use of analgesics.
The severe musculoskeletal pain may occur within days, months, or years after starting a bisphosphonate. Some patients have reported complete relief of symptoms after discontinuing the bisphosphonate, whereas others have reported slow or incomplete resolution. The risk factors for and incidence of severe musculoskeletal pain associated with bisphosphonates are unknown.
This severe musculoskeletal pain is in contrast to the acute phase response characterized by fever, chills, bone pain, myalgias, and arthralgias that sometimes accompanies initial administration of intravenous bisphosphonates and may occur with initial exposure to once-weekly or once-monthly doses of oral bisphosphonates. The symptoms related to the acute phase response tend to resolve within several days with continued drug use.
Healthcare professionals should consider whether bisphosphonate use might be responsible for severe musculoskeletal pain in patients who present with these symptoms and consider temporary or permanent discontinuation of the drug.
This information reflects FDA's current analysis of data available to FDA concerning this drug. FDA intends to update this when additional information or analyses become available.
FDA ALERT [1/7/2008] - FDA is highlighting the possibility of severe and sometimes incapacitating bone, joint, and/or muscle (musculoskeletal) pain in patients taking bisphosphonates. Although severe musculoskeletal pain is included in the prescribing information for all bisphosphonates, the association between bisphosphonates and severe musculoskeletal pain may be overlooked by healthcare professionals, delaying diagnosis, prolonging pain and/or impairment, and necessitating the use of analgesics.
The severe musculoskeletal pain may occur within days, months, or years after starting a bisphosphonate. Some patients have reported complete relief of symptoms after discontinuing the bisphosphonate, whereas others have reported slow or incomplete resolution. The risk factors for and incidence of severe musculoskeletal pain associated with bisphosphonates are unknown.
This severe musculoskeletal pain is in contrast to the acute phase response characterized by fever, chills, bone pain, myalgias, and arthralgias that sometimes accompanies initial administration of intravenous bisphosphonates and may occur with initial exposure to once-weekly or once-monthly doses of oral bisphosphonates. The symptoms related to the acute phase response tend to resolve within several days with continued drug use.
Healthcare professionals should consider whether bisphosphonate use might be responsible for severe musculoskeletal pain in patients who present with these symptoms and consider temporary or permanent discontinuation of the drug.
This information reflects FDA's current analysis of data available to FDA concerning this drug. FDA intends to update this when additional information or analyses become available.
Labels:
Actonel,
alert,
Aredia,
bisphosphonates,
bone pain,
Boniva,
Didronel,
Fosamax,
joint pain,
muscle pain,
Reclast,
Skelid,
Zometa
Subscribe to:
Comments (Atom)
Wandering Skeleton

Artist: Joel Hoekstra
Osteoporotic Bone
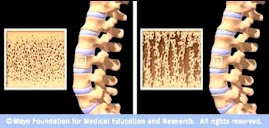
Source: www.mayoclinic.com
How Strontium Builds Bones
Strontium is a mineral that tends to accumulate in bone. Studies have shown that oral doses of strontium are a safe and effective way to prevent and reverse osteoporosis. Doses of 680 mg per day appear to be optimal. See my "For More Information About Strontium" links section.
Osteoporosis is caused by changes in bone production. In healthy young bones there is a constant cycle of new bone growth and bone removal. With age, more bone is removed and less new bone is produced. The bones become less dense and thus more fragile.
Scientists believe that strontium works in two ways. It may stimulate the replication of pre-osteoblasts, leading to an increase in osteoblasts (cells that build bone). Strontium also directly inhibits the activity of osteoclasts (cells that break down bone). The result is stronger bones.
When taking strontium, be sure to take 1200 mg calcium, 1000 IU vitamin D3, and 500 mg magnesium daily. It is best to take strontium late at night on an empty stomach. Calcium and strontium may compete with each other for absorption if taken together.
Osteoporosis is caused by changes in bone production. In healthy young bones there is a constant cycle of new bone growth and bone removal. With age, more bone is removed and less new bone is produced. The bones become less dense and thus more fragile.
Scientists believe that strontium works in two ways. It may stimulate the replication of pre-osteoblasts, leading to an increase in osteoblasts (cells that build bone). Strontium also directly inhibits the activity of osteoclasts (cells that break down bone). The result is stronger bones.
When taking strontium, be sure to take 1200 mg calcium, 1000 IU vitamin D3, and 500 mg magnesium daily. It is best to take strontium late at night on an empty stomach. Calcium and strontium may compete with each other for absorption if taken together.
For More Information about Strontium
- A Dose-response Study With Strontium Malonate
- A Review of the latest insights into the mechanism of action of strontium in bone
- Antifracture Efficacy Over 10 Years With Strontium Ranelate
- Combination of Micronutrients for Bone (COMB) Study: Bone Density after Micronutrient Intervention
- Echolight REMS Scan of Young, Normal Female
- Effect of bone strontium on BMD measurements
- Effect of Lumbar Scoliosis on DXA Results
- Effects of SrR on Calcium Metabolism
- Effects of strontium ions on growth and dissolution of hydroxyapatite and on bone mineral detection
- Influence of strontium on bone mineral density and bone mineral content measurements by dual X-ray absorptiometry
- Interpretation of BMD Scans in Patients Stopping Strontium
- Melatonin-micronutrients Osteopenia Treatment Study (MOTS)
- National Osteoporosis Foundation
- Osteoporosis And Bone Physiology
- Post-Marketing Assessment of the Safety of Strontium Ranelate
- PubMed Abstract On The SOTI Study
- PubMed Abstract On The TROPOS Study
- Strontium ranelate Aristo
- Strontium Ranelate For Spinal Osteoarthritis
- Strontium: Breakthrough Against Osteoporosis
- Summary Safety Review - Strontium
- The Effects of Strontium Ranelate on the Risk of Vertebral Fracture in Women with Postmenopausal Osteoporosis
- The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment
- Thirteen Key Diagnostic Tests