“Strontium ranelate is a new
anti-osteoporotic treatment. On bone biopsies collected from humans receiving
long-term treatment over 5 yr, it has been shown that strontium ranelate has
good bone safety and better results than placebo on 3D microarchitecture.
Hence, these effects may explain the decreased fracture rate.”
“Introduction: Strontium ranelate's mode of action involving dissociation
of bone formation and resorption was shown in preclinical studies and could
explain its antifracture efficacy in humans.”
“Materials and Methods: One hundred forty-one transiliac bone biopsies were
obtained from 133 postmenopausal osteoporotic women: 49 biopsies after 1–5 yr
of 2 g/d strontium ranelate and 92 biopsies at baseline or after 1–5 yr of
placebo.”
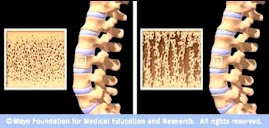
“Results and Conclusions: Histomorphometry provided a 2D demonstration of the bone
safety of strontium ranelate, with significantly higher mineral apposition rate
(MAR) in cancellous bone (+9% versus control, p = 0.019) and borderline
higher in cortical bone (+10%, p = 0.056). Osteoblast surfaces were
significantly higher (+38% versus control, p = 0.047). 3D analysis of
3-yr biopsies with treatment (20 biopsies) and placebo (21 biopsies) using μCT
showed significant changes in microarchitecture with, in the strontium ranelate
group, higher cortical thickness (+18%, p = 0.008) and trabecular number
(+14%, p = 0.05), and lower structure model index (−22%, p =
0.01) and trabecular separation (−16%, p = 0.04), with no change in
cortical porosity. The changes in 3D microarchitecture may enhance bone
biomechanical competence and explain the decreased fracture rate with strontium
ranelate.”
Figure 1. 3D microstructure from μCT of transiliac bone biopsies from postmenopausal osteoporotic women receiving strontium ranelate (2 g/d) or placebo for 3 yr.
Figure 1. 3D microstructure from μCT of transiliac bone biopsies from postmenopausal osteoporotic women receiving strontium ranelate (2 g/d) or placebo for 3 yr.


No comments:
Post a Comment