Cessation
of marketing of Protelos/Osseor: Extract of the letter sent to European
Medicine Agency (EMA) and national European Agencies on 10 February 2017
14/03/2017
Similar letters, adapted to local
regulations, have been sent to all countries worldwide where Protelos/Osseor®
is marketed
PROTELOS/OSSEOR® - Cessation of
marketing
On 21 September 2004,
Protelos/Osseor® (strontium ranelate), centrally authorised
medicinal product, was granted a Marketing Authorisation by the European
Commission for the European Union (EU).
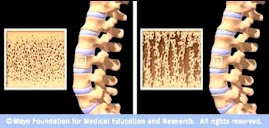
Protelos/Osseor® is
indicated in the treatment of severe osteoporosis in postmenopausal women and
in adult men at high risk of fracture, for whom treatment with other medicinal
products approved for the treatment of osteoporosis is not possible due to, for
example, contraindications or intolerance.
Les Laboratoires Servier hereby notify the cease of the
marketing, permanently, in the European countries where it is currently
marketed.
This worldwide and strategic decision is taken for commercial reasons based on the following grounds:
This worldwide and strategic decision is taken for commercial reasons based on the following grounds:
- The restricted indication/limited use of Protelos/Osseor®,
- The continuous decrease of patients treated with Protelos/Osseor®.
Les Laboratoires Servier will cease the distribution of
Protelos/Osseor® in August 2017.
Servier, founded in 1954, is the
first independent pharmaceutical group. We are present in 140 countries, with
more than 21 000 employees, including close to 3000 in R&D.
My note: Those who will no longer be prescribed strontium ranelate can buy nonprescription strontium citrate.


10 comments:
Wasn't there also a concern with the cardiovascular risk associated with long term use of strontium ranelate?
To: Eliot W. Collins,
Yes, there was an increased risk of nonfatal heart attacks in people with a history of cardiac problems. That finding precipitated the limitations placed on prescribing SR. The decrease in prescriptions caused Servier to stop marketing SR in Europe.
Hi BoneLady. I just ordered the strontium that you recommended. I am only 51. I have pretty severe OP in my spine. Can you email me and we possibly talk? I am Carol in Phoenix, AZ. cselman2@cox.net is my email.
Hi! BirdBones/Carol,
You can ask me anything you want via this blog, and I will answer you. Others may be interested in reading your questions and comments and my replies.
I hope the strontium citrate works well for your osteoporosis of the spine.
Best wishes,
BoneLady
Have you noted that a study of curcumin (turmeric) combined with soy lecithin has positively impacted bone development? I first noticed a British news article at http://www.dailymail.co.uk/health/article-4491740/Worried-osteoporosis-turmeric.html. A more detailed version of the study seems to be located at http://www.europeanreview.org/wp/wp-content/uploads/1684-1689-Curcumin-based-supplementation-in-osteopenia.pdf . It is of note that plain turmeric is not effective as there is little bioavailability. Combining with soy lecithin appears to allow much greater bioavailability. The version utilized in this study was Meriva. I'd be interested in your thoughts.
Hi BoneLady,
Since I'm new to your site, would you please tell me how your bone density is doing these days in 2017?? I'm considering taking strontium citrate but want to hear more about a real-life success story first.
Thanks in advance,
Kathy
Hi Kathy,
My latest results were posted on my blog on August 4, 2016. The following URL will take you to that post, which shows my bone density is in the upper range of osteopenia.
http://strontiumforbones.blogspot.com/2016/08/my-dxa-scan-on-august-4-2016.html
To Anonymous Poster of 9/22/17:
The study you referred to is a small (57 subjects), short (24 weeks), preliminary study on Thorne Research Meriva,a curcumin with soy. Thorne now has a soy-free Meriva.
It's too early to reach conclusions about curcumin to prevent and manage osteopenia, but curcumin is an anti-inflammatory compound with many uses.
I just stumbled across the incidence of serious cardiovascular events with the use of the bisphosphonate alendronate: it's a staggering 1.9%, compared with the 1.6% that vilified strontium ranelate. https://www.genengnews.com/gen-news-highlights/fda-rejects-amgen-ucb-osteoporosis-candidate-evenity/81254662?q=osteoporosis
Strontium citrate continues to be a great choice.
Jane B.
Thanks for the link. I agree that strontium citrate continues to be a great choice. I've been on a full dose (680 mg strontium/day) for nearly 10 years with no problems associated with it.
Post a Comment