Study data showed a beneficial effect in preventing fractures, including in patients at high risk of fracture. Available data do not show evidence of an increased cardiovascular risk with Protelos/Osseor in patients who did not have a history of heart or circulatory problems.
Human Skeleton

WELCOME TO STRONTIUM FOR BONES BLOG
Have you experienced negative, and even dangerous, side effects from Fosamax (alendronate), Boniva (ibandronate), Actonel (risedronate), Reclast (zoledronic acid), Prolia (denosumab), Forteo (teriparatide), Tymlos (abaloparatide), or other drugs prescribed for osteoporosis? If you have, then rest assured there is a safe, effective treatment for this condition. Strontium, primarily in the form of strontium citrate, is taken orally once a day.
Visitors to my blog can leave comments or ask questions and can remain anonymous, if they wish. Their comments are relayed to my g-mail inbox. Below each post, the number of comments for that post is cited and underlined because it is a link. By clicking on that link below any post, a window opens so that a visitor can leave a comment. Ideally, visitors leave comments on posts most relevant to their comments. All comments to my posts are moderated by me.
Browse the posts and visit the link library of references.
Visitors to my blog can leave comments or ask questions and can remain anonymous, if they wish. Their comments are relayed to my g-mail inbox. Below each post, the number of comments for that post is cited and underlined because it is a link. By clicking on that link below any post, a window opens so that a visitor can leave a comment. Ideally, visitors leave comments on posts most relevant to their comments. All comments to my posts are moderated by me.
Browse the posts and visit the link library of references.
Friday, February 21, 2014
Strontium Ranelate to Remain Available in EU
As of February 21, 2014, the European Medicines Agency
(EMA) has concluded its review of strontium ranelate (Protelos/Osseor). The EMA is
recommending the medication remain available in the European Union (EU) but further restricting its
use to patients who cannot be treated with other medicines approved for
osteoporosis due to contraindications or intolerance. These patients should continue
to be evaluated regularly by their doctor, and treatment should be stopped if
patients develop heart or circulatory problems. Patients with established,
current or past history of ischemic heart disease, peripheral arterial disease
and/or cerebrovascular disease, or those with uncontrolled hypertension should
not use the medicine.
Study data showed a beneficial effect in preventing fractures, including in patients at high risk of fracture. Available data do not show evidence of an increased cardiovascular risk with Protelos/Osseor in patients who did not have a history of heart or circulatory problems.
Study data showed a beneficial effect in preventing fractures, including in patients at high risk of fracture. Available data do not show evidence of an increased cardiovascular risk with Protelos/Osseor in patients who did not have a history of heart or circulatory problems.
Monday, February 10, 2014
Two New Studies Show Strontium Ranelate Not Associated with Increased Risk of MI
The safety of all centrally registered drugs is closely monitored by the European Medicines Agency (EMA) through a new committee, the Pharmacovigilance Risk Assessment Committee (PRAC), which was launched in October 2012. The procedures include regular submission of periodic safety update reports (PSURs).
In November 2012, the PSUR for strontium ranelate, which encompassed a number of new randomized clinical trials, included an updated assessment of the overall safety of the treatment and was submitted to the PRAC. The overall safety analyses showed an increased cardiovascular risk in patients treated with strontium ranelate. This ongoing process has led to a label change, and, in order to mitigate the cardiovascular risk, strontium ranelate is now contraindicated in patients with a history of cardiovascular disease, i.e. in patients with a history of ischemic heart disease, peripheral artery disease, and/or cerebrovascular disease and in those with uncontrolled hypertension.
In the light of these procedures, the results of two new studies were published together online on December 10, 2013, in Osteoporosis International. Both constitute retrospective observational studies conducted in databases of electronic healthcare records and were set up to analyze the cardiovascular risk associated with the prescription of strontium ranelate in real-life clinical practice in the UK and in Denmark.
The results of the studies are consistent on three points. First, observational data do NOT indicate that the use of strontium ranelate was associated with a significant increase in myocardial infarction (MI). Cooper et al. compared the risk of ischemic cardiac events in postmenopausal osteoporotic women who were currently receiving treatment with strontium ranelate—or had received it in the past—with the risk in those who had never received strontium ranelate. Current use or past use of strontium ranelate was not associated with any significant increase in the risk for three cardiovascular events: first MI, hospitalization with MI, or cardiovascular death. Abrahamsen et al. reported that the risk for MI in men and postmenopausal women was not significantly elevated, though they did find a very borderline result for stroke and cardiovascular death and a significant increase in risk for all-cause mortality.
Second, both studies highlighted substantial differences in patient profile of users of strontium ranelate compared with users of other osteoporosis treatments. Strontium ranelate patients are generally older and have more severe osteoporosis and a longer duration of disease. They also have more comorbidities, notably those related to elevated cardiovascular risk, such as cardiac failure (22 % in the Danish study), peripheral vascular disease (6 %), and cerebrovascular disease (11 %), with a combined prevalence of ischemic heart disease, peripheral vascular disease, and cerebrovascular disease of 19 % in women and 30 % in men. The cases of ischemic cardiac events in the UK study were also at substantially higher risk compared with the controls, with higher rates of history of hospitalization for MI (12 versus 4 %), ischemic heart disease (71 versus 24 %), peripheral artery disease (18 versus 7 %), and cerebrovascular disease (23 versus 15 %). This is a significant finding for clinical practice: the majority of cases of MI occurred in patients who would not be treated with the agent according to the new contraindications for strontium ranelate.
Third, while both studies are highly robust, neither managed to properly address the major challenge of the potential channeling bias by which more fragile or severe patients are being switched to strontium ranelate. Clearly, further research is warranted with appropriate handling of the remaining bias for a more complete evaluation of risk.
The role of the clinician is to select the best treatment for the patient’s profile and individual therapeutic objective, which should remain the prevention of osteoporotic fracture. By strictly applying the new contraindications for strontium ranelate, we can hope to achieve our primary goal of treating disease, preventing osteoporotic fracture, while markedly reducing the risk for side effects.
http://link.springer.com/article/10.1007/s00198-013-2583-3/fulltext.html
In November 2012, the PSUR for strontium ranelate, which encompassed a number of new randomized clinical trials, included an updated assessment of the overall safety of the treatment and was submitted to the PRAC. The overall safety analyses showed an increased cardiovascular risk in patients treated with strontium ranelate. This ongoing process has led to a label change, and, in order to mitigate the cardiovascular risk, strontium ranelate is now contraindicated in patients with a history of cardiovascular disease, i.e. in patients with a history of ischemic heart disease, peripheral artery disease, and/or cerebrovascular disease and in those with uncontrolled hypertension.
In the light of these procedures, the results of two new studies were published together online on December 10, 2013, in Osteoporosis International. Both constitute retrospective observational studies conducted in databases of electronic healthcare records and were set up to analyze the cardiovascular risk associated with the prescription of strontium ranelate in real-life clinical practice in the UK and in Denmark.
The results of the studies are consistent on three points. First, observational data do NOT indicate that the use of strontium ranelate was associated with a significant increase in myocardial infarction (MI). Cooper et al. compared the risk of ischemic cardiac events in postmenopausal osteoporotic women who were currently receiving treatment with strontium ranelate—or had received it in the past—with the risk in those who had never received strontium ranelate. Current use or past use of strontium ranelate was not associated with any significant increase in the risk for three cardiovascular events: first MI, hospitalization with MI, or cardiovascular death. Abrahamsen et al. reported that the risk for MI in men and postmenopausal women was not significantly elevated, though they did find a very borderline result for stroke and cardiovascular death and a significant increase in risk for all-cause mortality.
Second, both studies highlighted substantial differences in patient profile of users of strontium ranelate compared with users of other osteoporosis treatments. Strontium ranelate patients are generally older and have more severe osteoporosis and a longer duration of disease. They also have more comorbidities, notably those related to elevated cardiovascular risk, such as cardiac failure (22 % in the Danish study), peripheral vascular disease (6 %), and cerebrovascular disease (11 %), with a combined prevalence of ischemic heart disease, peripheral vascular disease, and cerebrovascular disease of 19 % in women and 30 % in men. The cases of ischemic cardiac events in the UK study were also at substantially higher risk compared with the controls, with higher rates of history of hospitalization for MI (12 versus 4 %), ischemic heart disease (71 versus 24 %), peripheral artery disease (18 versus 7 %), and cerebrovascular disease (23 versus 15 %). This is a significant finding for clinical practice: the majority of cases of MI occurred in patients who would not be treated with the agent according to the new contraindications for strontium ranelate.
Third, while both studies are highly robust, neither managed to properly address the major challenge of the potential channeling bias by which more fragile or severe patients are being switched to strontium ranelate. Clearly, further research is warranted with appropriate handling of the remaining bias for a more complete evaluation of risk.
The role of the clinician is to select the best treatment for the patient’s profile and individual therapeutic objective, which should remain the prevention of osteoporotic fracture. By strictly applying the new contraindications for strontium ranelate, we can hope to achieve our primary goal of treating disease, preventing osteoporotic fracture, while markedly reducing the risk for side effects.
http://link.springer.com/article/10.1007/s00198-013-2583-3/fulltext.html
Subscribe to:
Comments (Atom)
Wandering Skeleton

Artist: Joel Hoekstra
Osteoporotic Bone
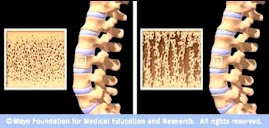
Source: www.mayoclinic.com
How Strontium Builds Bones
Strontium is a mineral that tends to accumulate in bone. Studies have shown that oral doses of strontium are a safe and effective way to prevent and reverse osteoporosis. Doses of 680 mg per day appear to be optimal. See my "For More Information About Strontium" links section.
Osteoporosis is caused by changes in bone production. In healthy young bones there is a constant cycle of new bone growth and bone removal. With age, more bone is removed and less new bone is produced. The bones become less dense and thus more fragile.
Scientists believe that strontium works in two ways. It may stimulate the replication of pre-osteoblasts, leading to an increase in osteoblasts (cells that build bone). Strontium also directly inhibits the activity of osteoclasts (cells that break down bone). The result is stronger bones.
When taking strontium, be sure to take 1200 mg calcium, 1000 IU vitamin D3, and 500 mg magnesium daily. It is best to take strontium late at night on an empty stomach. Calcium and strontium may compete with each other for absorption if taken together.
Osteoporosis is caused by changes in bone production. In healthy young bones there is a constant cycle of new bone growth and bone removal. With age, more bone is removed and less new bone is produced. The bones become less dense and thus more fragile.
Scientists believe that strontium works in two ways. It may stimulate the replication of pre-osteoblasts, leading to an increase in osteoblasts (cells that build bone). Strontium also directly inhibits the activity of osteoclasts (cells that break down bone). The result is stronger bones.
When taking strontium, be sure to take 1200 mg calcium, 1000 IU vitamin D3, and 500 mg magnesium daily. It is best to take strontium late at night on an empty stomach. Calcium and strontium may compete with each other for absorption if taken together.
For More Information about Strontium
- A Dose-response Study With Strontium Malonate
- A Review of the latest insights into the mechanism of action of strontium in bone
- Antifracture Efficacy Over 10 Years With Strontium Ranelate
- Combination of Micronutrients for Bone (COMB) Study: Bone Density after Micronutrient Intervention
- Echolight REMS Scan of Young, Normal Female
- Effect of bone strontium on BMD measurements
- Effect of Lumbar Scoliosis on DXA Results
- Effects of SrR on Calcium Metabolism
- Effects of strontium ions on growth and dissolution of hydroxyapatite and on bone mineral detection
- Influence of strontium on bone mineral density and bone mineral content measurements by dual X-ray absorptiometry
- Interpretation of BMD Scans in Patients Stopping Strontium
- Melatonin-micronutrients Osteopenia Treatment Study (MOTS)
- National Osteoporosis Foundation
- Osteoporosis And Bone Physiology
- Post-Marketing Assessment of the Safety of Strontium Ranelate
- PubMed Abstract On The SOTI Study
- PubMed Abstract On The TROPOS Study
- Strontium ranelate Aristo
- Strontium Ranelate For Spinal Osteoarthritis
- Strontium: Breakthrough Against Osteoporosis
- Summary Safety Review - Strontium
- The Effects of Strontium Ranelate on the Risk of Vertebral Fracture in Women with Postmenopausal Osteoporosis
- The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment
- Thirteen Key Diagnostic Tests